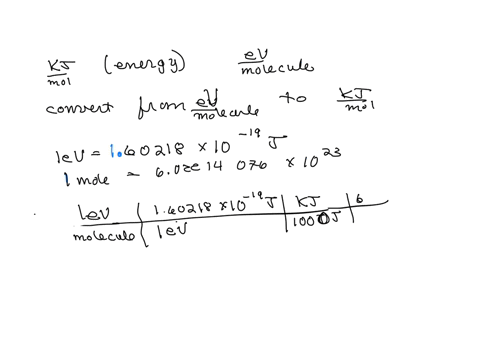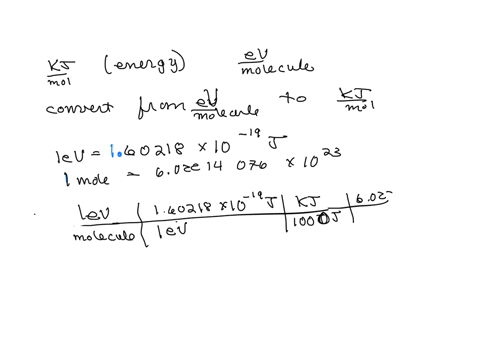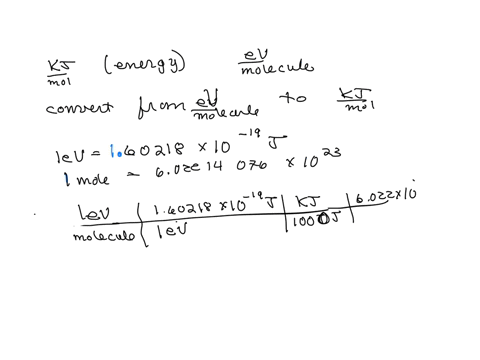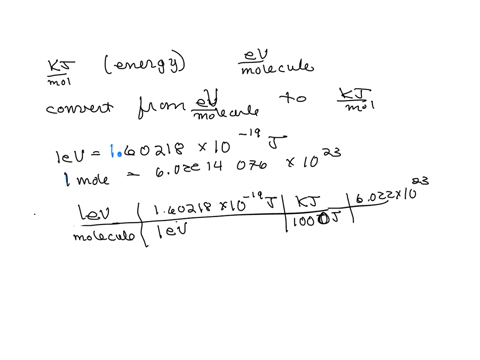SOLVED: Calculate (a) the energy (in eV) of a 5.3-Ã… X-ray photon and (b) the energy (kJ/mol) of a 530-nm photon of visible radiation. Given the following constants and unit conversion factors:
Magnesium has the firšt and second ionizatio: potential 7.646 and 15.035 ev respectively. What is theof energy required to convert all the magnesium atoms to Mg2+ ions present in 24 mg to

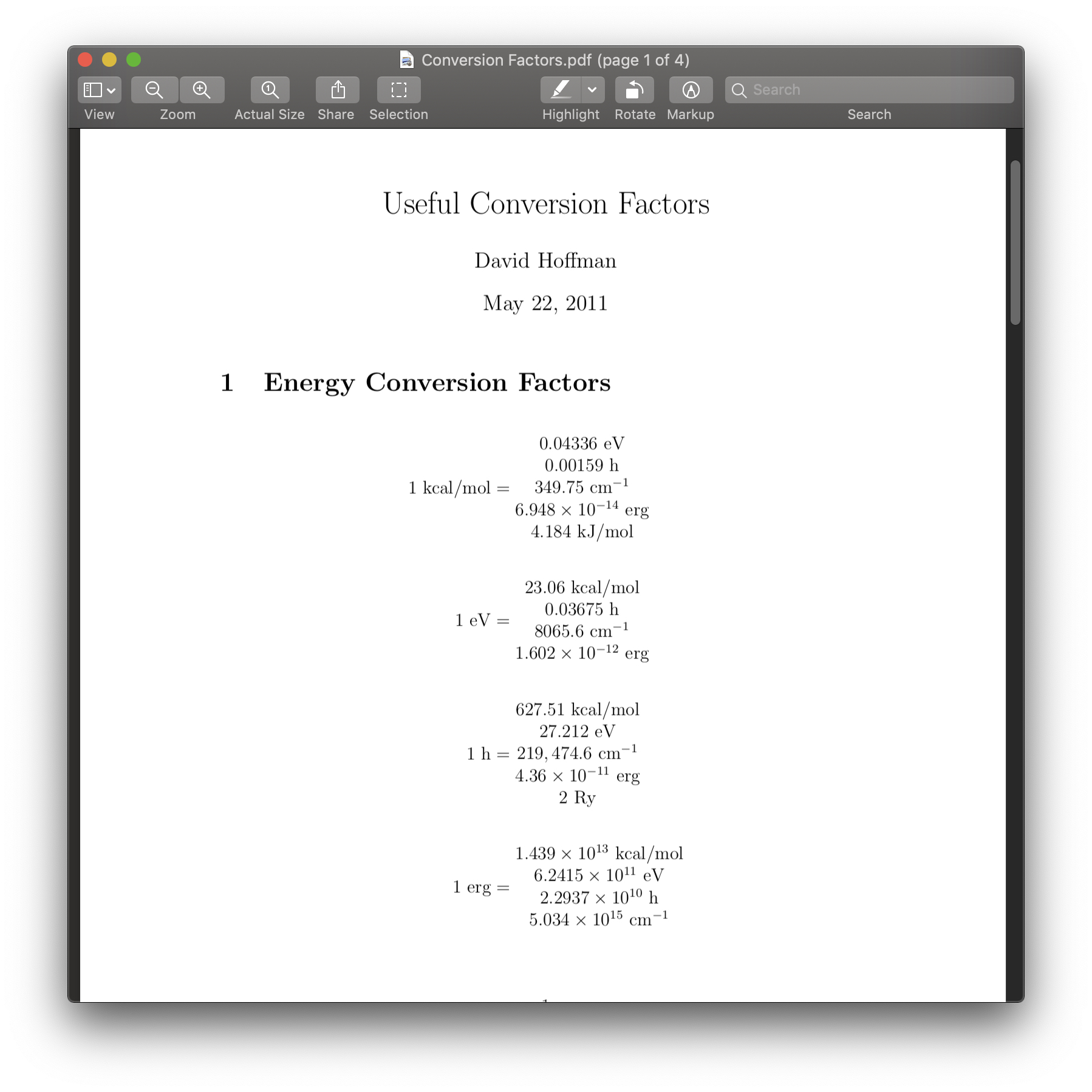
SOLVED: Convert these two atomic units (a.u.) into kJ/mol by using 2625 kJ/ mol = 1 a.u. -78.7939 kJ/mol -78.7838378 kJ/mol Please show the work.

Thermodymanics Lecture 3 8/31/2004. Units Energy Ultimate source of energy is the sun E = h 57 Kcal/mol of photons green light or 238 KJ/mol 1 cal = - ppt download
the energy required to convert all atoms presrent in 1.2g magnesium to magmnesium to mg^2+ ions if lE,and lE_2 of magnesium are 120kj mol^ 1 and 240 kj mol^ 1 respectivel

1 MODELING MATTER AT NANOSCALES 6. The theory of molecular orbitals for the description of nanosystems (part II) The Hartree-Fock method applied. - ppt download

1 ev =???? kj/mole in full details | 1 ev me kitne killo joule hote hai| 1ev me Kitna joule hota hai - YouTube

Table 2 from A mathematical introduction to Hartree-Fock SCF methods in Quantum Chemistry | Semantic Scholar

SOLVED: (a)how much energy in KJ/mol is released when an electron makes a transition from n=5 to n=2 in a hydrogen atom?

CHM 4444, Advanced Inorganic Chemistry. Chapters 1, Atomic structure 2, Molecular structure & bonding 3, Structures of simple solids 6, Molecular symmetry. - ppt download